Contact Us
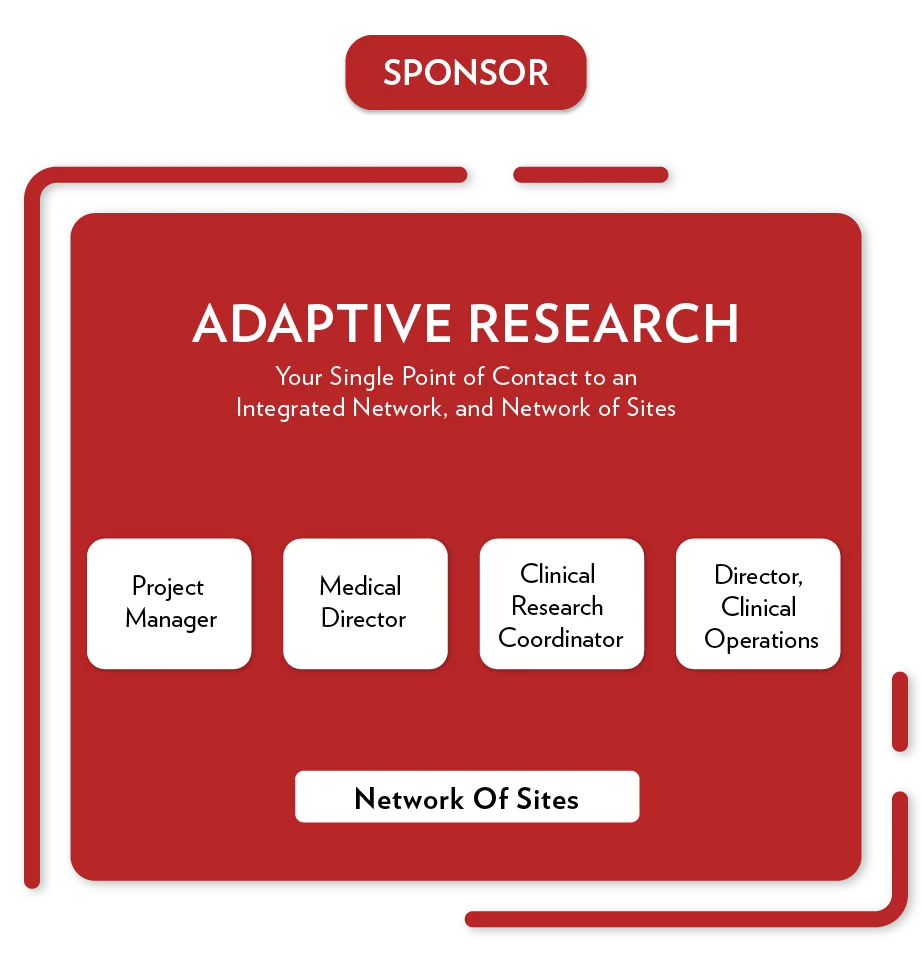
Solutions
Solutions

Disrupting the Clinical Trial Landscape
- Democratizing clinical trials by partnering with community physicians
- Utilizing digital technologies to expand access and drive efficiency
- Offering a wide range of solutions and services for Sponsors, Contract Research Organizations and Community Practices
- Trial Management Software, Regulatory Affairs
Solving a Clinical Need
Majority of patients lack access to clinical trials
Only 5% of eligible patients participate in clinical trials
85% of patients receive healthcare and treatment outside of academic or specialized research centers
More than 50% of trials are terminated due to low enrollment
Unavailability of pharmaceutical solutions and technologies to patients in need
Results are underpowered
Sites and Physicians who are experienced to participate in proof of concept studies. These sites often help to speed up Phase-I development that includes efficacy, and bio-distribution.
Services to meet the gap between development and commercialization.
Sites and Physicians that meet the needs of the increased patient population, and service to support global research and advancement for life-saving therapies
First-in-human/Phase-I
Sites and Physicians who are experienced to participate in proof of concept studies. These sites often help to speed up Phase-I development that includes efficacy, and bio-distribution.
Phase II-III
Services to meet the gap between development and commercialization.
Late Phase
Sites and Physicians that meet the needs of the increased patient population, and service to support global research and advancement for life-saving therapies
Sites and Physicians who are experienced to participate in proof of concept studies. These sites often help to speed up Phase-I development that includes efficacy, and bio-distribution.
Services to meet the gap between development and commercialization.
Sites and Physicians that meet the needs of the increased patient population, and service to support global research and advancement for life-saving therapies
More than 50% of trials are terminated due to low enrollment
Unavailability of cutting-edge drugs and technologies to patients in need
Results are underpowered
Solving a Clinical Need
Majority of patients lack access to clinical trials
Only 5% of eligible patients participate in clinical trials
85% of patients receive healthcare and treatment outside of academic or specialized research centers
More than 50% of trials are terminated due to low enrollment
Unavailability of pharmaceutical solutions and technologies to patients in need
Results are underpowered
Sites and Physicians who are experienced to participate in proof of concept studies. These sites often help to speed up Phase-I development that includes efficacy, and bio-distribution.
Services to meet the gap between development and commercialization.
Sites and Physicians that meet the needs of the increased patient population, and service to support global research and advancement for life-saving therapies
First-in-human/Phase-I
Sites and Physicians who are experienced to participate in proof of concept studies. These sites often help to speed up Phase-I development that includes efficacy, and bio-distribution.
Phase II-III
Services to meet the gap between development and commercialization.
Late Phase
Sites and Physicians that meet the needs of the increased patient population, and service to support global research and advancement for life-saving therapies
Sites and Physicians who are experienced to participate in proof of concept studies. These sites often help to speed up Phase-I development that includes efficacy, and bio-distribution.
Services to meet the gap between development and commercialization.
Sites and Physicians that meet the needs of the increased patient population, and service to support global research and advancement for life-saving therapies
More than 50% of trials are terminated due to low enrollment
Unavailability of cutting-edge drugs and technologies to patients in need
Results are underpowered
Providing Clinical Operations Expertise
Highly Experienced Clinical Research Personnel
GCP-trained Research Network
Trial management software
Regulatory affairs
Quality & Compliance
Medical Science
Services
Sponsors/CROs
Full-Service Operationalization
- Tapping into the “untapped” pool
- Understanding competing priorities
- Identifying gaps and opportunities
- Efficient and thorough clinical operations (contracts, budgets, IRB, etc.)
- Convenience: Single point of contact for administration, monitoring, audits
- Work alongside sponsor CROs
Physicians
Full-service Operationalization
- Technology enabled
- Operational efficiencies Contract
- Consents
- Screening
- Enrollment
- Data collection
- Closeout activities
- Technology enabled
- Operational efficiencies Contracts, consents, screening, enrollment, data collection, data entry, closeout activities
- Clinical trial expertise
- Clinical trial related finance & budgeting
- Research support staff
- FDA & IRB regulations
- Business development
Physicians Role
- Clinical judgment
- Physicians Investigator
- Patient identification
- Physicians assessments
- Clinical procedures
Physicians Success is our Success
- Physicians driven organization
- Physicians are customers, not service providers
- Physicians and patient satisfaction held in the highest regard
- Build relationships to ensure high stickiness from physicians in our network
Clinical Development/Sites
Full-service Operationalization
- Site selection and onboarding
- Administration and regulatory support
- Financial support
- Digital technologies
- Marginalized populations
- Clinical operations management
Capabilities
Current Therapeutic Areas
- A one-stop shop for multiple specialty indications
- Foundation-sponsored research experience (MJFF and Parkinson’s Foundation)
- Experience interacting with government entities to raise awareness of issues
- Extensive clinical research expertise including in rare/small population diseases
